
Patients whose devices were fragmented also underwent echocardiography and cardiac computed tomography. The 80 underwent a fluoroscopy to determine the structural integrity of the filter and investigate whether it had broken or was still intact. William Nicholson at York Hospital in Pennsylvania examined 80 patients who had been implanted with Bard IVC filters from 2004 – 2009. Known long term risks associated with IVC filters include but are not limited to lower limb deep vein thrombosis (DVT), filter fracture, filter migration, filter embolization and IVC perforation.”Ī research team led by Dr. “The FDA is concerned that these retrievable IVC filters, intended for short-term placement, are not always removed once a patient’s risk for PE subsides. Since 2005, the FDA said it has received “921 device adverse event reports involving IVC filters, 328 for device migration 146 for embolizations (detachment of device components) 70 for perforation of the IVC 56 for filter fracture.” Some of these events led to problems for patients, according to FDA, which also said the events could be related to a retrievable filter remaining in patients’ bodies beyond the time when pulmonary embolism (PE) risk subsided: The agency was concerned about doctors not retrieving IVC filters intended only for short-term placement, hence exposing patients to fractures and all the problems which can follow. FDA advised implanting doctors to remove the devices once PE risk subsides. ( See the PDF.) The FDA warned that IVC filters are meant for short-term use only, and only for patients at risk for pulmonary embolism. In August 2010, FDA issued an initial warning against leaving inferior vena cava filters implanted in patients for extended periods due to their potential to cause adverse health complications. The intended audience was implanting physicians and clinicians in charge of caring for patients with inferior vena cava (IVC) filters, as well as interventional radiologists and cardiologists, vascular surgeons, emergency room (trauma) and primary care physicians, bariatric and orthopedic surgeons. On May 6, 2014, the FDA updated its safety communication: “Removing Retrievable Inferior Vena Cava Filters” ( see PDF).

#Class actio suit on heart blood clot filters free#
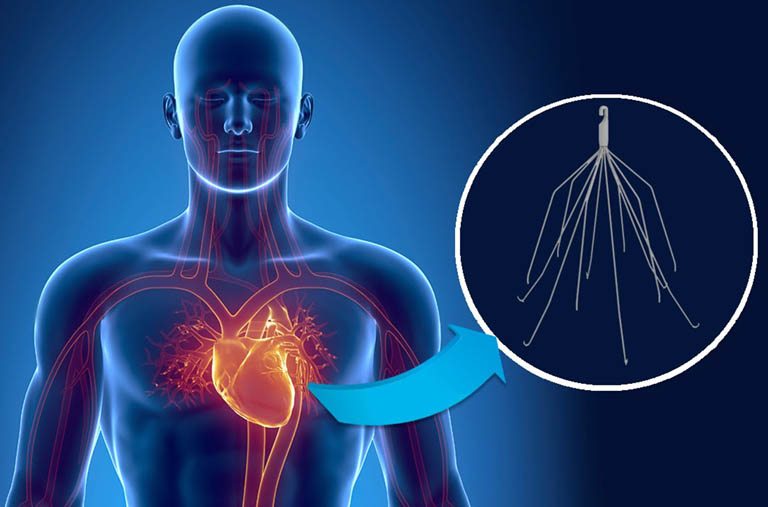
IVC Filter Profileĭo I have a Bard IVC Filter Lawsuit or Blood Clot Filter Lawsuit?Ĭontact an experienced IVC filter attorney at Matthews & Associates for a free legal consultation if you believe you may have cause to pursue a Bard IVC Filter Lawsuit or a Blood Clot Filter Lawsuit against the makers of a blood clot filter. Two vena cava transport blood through human beings: the inferior vena cava carries blood from the lower body the superior vena cava moves blood from the head, arms and upper body. The vena cava is a large vein that carries deoxygenated blood into the heart. Similar problems have been found with other IVC filters made by Cook Medical, Boston Scientific, B. Reports include 328 for device migration, 146 for embolizations (device component detachment), 70 for perforation of the IVC, 56 for filter fracture. FDA has received more than 1,000 reports of serious adverse events related to Bard IVC Filters. The FDA updated a 2010 safety communication in May 2014 regarding device removal complications. FDA Reports IVC Blood Clot Filter Injuries Please view our testimonials to see how we have helped others injured by dangerous medical devices and pharmaceutical drugs. If you have been injured by a blood clot filter, or had an IVC filter installed which migrated and could not or cannot be safely removed, contact an experienced IVC Filter Lawyer for a potential Bard IVC Filter Lawsuit, or a Bard Recovery Blood Clot Filter Lawsuit. Update: Philadelphia IVC Filter Case Filed Contact us for a free legal consultation. Our law firm is filing Bard IVC Filter Lawsuits and Cook IVC Filter Lawsuits nationwide. Other troublesome IVC blood clot filters include the Bard Eclipse, Bard Merdian, Bard Denali IVC. IVC filters most often seen in the litigation include the Bard Recovery filter, Bard G2 filter, Bard G2 Express filter, Cook Gunther Tulip filter, Cook Celect filter, Rex Medical Option filter. An IVC Filter Lawsuit Lawyer may be able to help with a free legal consultation if you or someone you know has been implanted with an IVC filter.

Bard Recovery IVC filters may even be fatally flawed. The Journal of the American Medical Association (JAMA) has reported that IVC blood clot filters show evidence of harm with no evidence of benefit. Benzene Sunscreen Recall – Leukemia, Blood Cancers.Boy Scouts of America Lawsuit | BSA Lawyer.Parkinson’s Lawsuit – Paraquat Pesticide.Infant Formula – (NEC) Necrotizing Enterocolitis.Camp Lejeune Water Contamination Attorneys.


 0 kommentar(er)
0 kommentar(er)
